About a century ago, the Swedish physical scientist Arrhenius proposed a law of classical chemistry that relates chemical reaction rate to temperature. According to the Arrhenius equation, chemical reaction are increasingly unlikely to occur as temperatures approach absolute zero, and at absolute zero (zero degrees Kelvin, or minus 273 degrees Celsius) reactions stop. However, recent experimental evidence reveals that although the Arrhenius equation is generally accurate in describing the kind of chemical reaction that occurs at relatively high temperatures, at temperatures closer to zero a quantum-mechanical effect known as tunneling comes into play; this effect accounts for chemical reactions that are forbidden by the principles of classical chemistry. Specifically, entire molecules can "tunnel" through the barriers of repulsive forces from other molecules and chemically react even though these molecules do not have sufficient energy, according to classical chemistry, to overcome the repulsive barrier.
The rate of any chemical reaction, regardless of the temperature at which it takes place, usually depends on a very important characteristic known as its activation energy. Any molecule can be imagined to reside at the bottom of a so-called potential well of energy. A chemical reaction corresponds to the transition of a molecule from the bottom of one potential well to the bottom of another. In classical chemistry, such a transition can be accomplished only by going over the potential barrier between the wells, the height of which remains constant and is called the activation energy of the reaction. In tunneling, the reacting molecules tunnel from the bottom of one to the bottom of another well without having to rise over the barrier between the two wells. Recently researchers have developed the concept of tunneling temperature: the temperature below which tunneling transitions greatly outnumber Arrhenius transitions, and classical mechanics gives way to its quantum counterpart.
This tunneling phenomenon at very low temperatures suggested my hypothesis about a cold prehistory of life: the formation of rather complex organic molecules in the deep cold of outer space, where temperatures usually reach only a few degrees Kelvin. Cosmic rays (high-energy protons and other particles) might trigger the synthesis of simple molecules, such as interstellar formaldehyde, in dark clouds of interstellar dust. Afterward complex organic molecules would be formed, slowly but surely, by means of tunneling. After I offered my hypothesis, Hoyle and Wickramasinghe argued that molecules of interstellar formaldehyde have indeed evolved into stable polysaccharides such as cellulose and starch. Their conclusions, although strongly disputed, have generated excitement among investigators such as myself who are proposing that the galactic clouds are the places where the prebiological evolution of compounds necessary to life occurred.
1.According to the passage, classical chemical reactions and tunneling reactions are alike in which of the following ways?
(A) In both types of reactions, reacting molecules have to rise over the barrier between the two wells.
(B) In both types of reactions, a transition is made from the bottom of one potential well to the bottom of another.
(C) In neither type of reaction does the height of the barrier between the wells remain constant.
(D) In neither type of reaction does the rate of a chemical reaction depend on its activation energy.
(E) In both types of reactions, reacting molecules are able to go through the barrier between the two wells.
2.The author's hypothesis concerning the cold prehistory of life would be most weakened if which of the following were true?
(A)Cosmic rays are unlikely to trigger the formation of simple molecules.
(B)Tunneling occurs only in a narrow band of temperatures around zero degrees Kelvin.
(C)The synthesis of interstellar formaldehyde can be activated by means other than cosmic rays.
(D)Simple molecules can be synthesized by means of tunneling.
(E)Classical chemical reactions do not occur at temperatures close to absolute zero.
3.Which of the following best describes the hypothesis of Hoyle and Wickramasinghe as it is presented in the passage?
(A) Cosmic rays can directly synthesize complex organic molecules.
(B) The galactic clouds are the places where prebiological evolution of compounds necessary to life occurred.
(C) Interstellar formaldehyde can be synthesized by tunneling.
(D) Molecules of interstellar formaldehyde can evolve into complex organic molecules.
(E) Complex organic molecules can be synthesized from stable polysaccharides such as cellulose and starch.
4.Which of the following best describes the organization of the first two paragraphs of the passage?
(A) The author cites a basic principle of classical chemistry and then describes the research from which that principle was developed.
(B) The author cites an apparent contradiction to the principles of classical chemistry and then explains the process of a chemical reaction to show there is in fact no contradiction.
(C) the author describes the role of heat in chemical reactions and then offers a detailed explanation of its function.
(D) The author presents a law of classical chemistry in order to introduce a kind of chemical reaction that differs from it and then explains the essential difference between the two.
(E) The author presents the fundamental rules of classical chemistry in order to introduce an explanation of a specific chemical reaction.
5. The author of the passage is primarily concerned with
(A) describing how the principles of classical chemistry were developed
(B) initiating a debate about the kinds of chemical reactions required for the development of life
(C) explaining how current research in chemistry may be related to broader biological concerns
(D) reconciling opposing theories about chemical reactions
(E) clarifying inherent ambiguities in the laws of classical chemistry
結構分析:
以下灰色部分是我認為不必再一開始抓主幹時候看的內容
About a century ago, the Swedish physical scientist Arrhenius proposed a law of classical chemistry that relates chemical reaction rate to temperature. According to the Arrhenius equation, chemical reaction are increasingly unlikely to occur as temperatures approach absolute zero, and at absolute zero (zero degrees Kelvin, or minus 273 degrees Celsius) reactions stop.(Arrhenius上面提過,補充說明用的跳過) However, recent experimental evidence reveals that although the Arrhenius equation is generally accurate in describing the kind of chemical reaction that occurs at relatively high temperatures(句內轉折別理他,看一半就好), at temperatures closer to zero a quantum-mechanical effect known as tunneling comes into play; this effect accounts for chemical reactions that are forbidden by the principles of classical chemistry(分號後方是補充說明用的別理他). Specifically, entire molecules can "tunnel" through the barriers of repulsive forces from other molecules and chemically react(前面的副詞Specifically表示特殊情況,還是一組補充說明) even though these molecules do not have sufficient energy, according to classical chemistry, to overcome the repulsive barrier.(強調句出現,邏輯跟前句一樣,所以還是跳過)
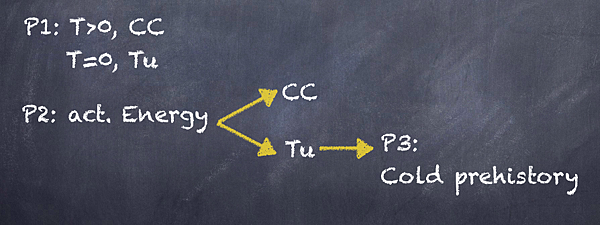
總結第一段:可以發現對於溫度的反應有所不同,T>0,經典化學,T~0,穿隧效應。
The rate of any chemical reaction, regardless of the temperature at which it takes place,(差入句忽略) usually depends on a very important characteristic known as its activation energy. Any molecule can be imagined to reside at the bottom of a so-called potential well of energy.(Any molecule,他跟主題句之間又另外岔開話題了,然後中間沒有轉折詞出現,為內容補充,再說any這個字本身就有分類的含義,在主題句的定義之下任何一個粒子會怎樣怎樣,這變成是細節來對主題展開,那既然是細節,就不多談拉,略) A chemical reaction corresponds to the transition of a molecule from the bottom of one potential well to the bottom of another. (本段主題是化學反應的『速度』喔,既然如此chemical reaction當然就是細節了,跳)In classical chemistry, such a transition can be accomplished only by going over the potential barrier between the wells, the height of which remains constant and is called the activation energy of the reaction. In tunneling, the reacting molecules tunnel from the bottom of one to the bottom of another well without having to rise over the barrier between the two wells. Recently researchers have developed the concept of tunneling temperature: the temperature below which tunneling transitions greatly outnumber Arrhenius transitions, and classical mechanics gives way to its quantum counterpart.
第二段:化學反應速率要關心到活化能,然後In classical chemistry,In tunneling,分門別類討論他們會發生什麼事情,一樣屬於細節,都先略過,這文章突然跳到速度的議題,然後速度又跟activation energy.互扯,如果再把剛剛的綠色線索In classical chemistry,In tunneling,給考量進來,表示作者又用另一種方式分類,這次基準就是活化能我們筆記成act.
This tunneling phenomenon at very low temperatures suggested my hypothesis about a cold prehistory of life: the formation of rather complex organic molecules in the deep cold of outer space, where temperatures usually reach only a few degrees Kelvin.(分號內容先放一邊) Cosmic rays (high-energy protons and other particles) might trigger the synthesis of simple molecules, such as interstellar formaldehyde, in dark clouds of interstellar dust. Afterward complex organic molecules would be formed, slowly but surely, by means of tunneling.(該名詞前方內容出現過,忽略)After I offered my hypothesis, Hoyle and Wickramasinghe argued that molecules of interstellar formaldehyde have indeed evolved into stable polysaccharides such as cellulose and starch(出現兩個人名舉例). Their conclusions, although strongly disputed, have generated excitement among investigators such as myself who are proposing that the galactic clouds are the places where the prebiological evolution of compounds necessary to life occurred.
第三段:經過刪減之後,由主題句看到,作者轉移話題了,原來他想用穿隧效應來假設他自己的觀點cold prehistory of life,最後下結論是激勵人心,這表示贊同啊。所以文章是一種類比法,用物理現象去類比生物反應,做題。
1.According to the passage, classical chemical reactions and tunneling reactions are alike in which of the following ways?
(A) In both types of reactions, reacting molecules have to rise over the barrier between the two wells.
(B) In both types of reactions, a transition is made from the bottom of one potential well to the bottom of another.
(C) In neither type of reaction does the height of the barrier between the wells remain constant.
(D) In neither type of reaction does the rate of a chemical reaction depend on its activation energy.
(E) In both types of reactions, reacting molecules are able to go through the barrier between the two wells.
解題:
兩個相同點,由結構圖看只有P2的內容是分支出去兩個反應,表是那個act ennergy是『共同』座標,基準點必有共同處,因此考點在第二段,所以找到解釋activation energy.的句子,也就是主題句之下與分類成In classical chemistry,In tunneling,兩內容中間的兩句,就是考點了,
Any molecule can be imagined to reside at the bottom of a so-called potential well of energy. A chemical reaction corresponds to the transition of a molecule from the bottom of one potential well to the bottom of another.
從甲地進乙地出,選(B)
2.The author's hypothesis concerning the cold prehistory of life would be most weakened if which of the following were true?
(A)Cosmic rays are unlikely to trigger the formation of simple molecules.
(B)Tunneling occurs only in a narrow band of temperatures around zero degrees Kelvin.
(C)The synthesis of interstellar formaldehyde can be activated by means other than cosmic rays.
(D)Simple molecules can be synthesized by means of tunneling.
(E)Classical chemical reactions do not occur at temperatures close to absolute zero.
解題:
由題目標定到第三段,並且找到跟the cold prehistory of life相關的句子,考的很像邏輯單題,所以來對這個地方做logic book style:
This tunneling phenomenon at very low temperatures suggested my hypothesis about a cold prehistory of life: the formation of rather complex organic molecules in the deep cold of outer space, where temperatures usually reach only a few degrees Kelvin. Cosmic rays (high-energy protons and other particles) might trigger the synthesis of simple molecules, such as interstellar formaldehyde, in dark clouds of interstellar dust. Afterward complex organic molecules would be formed, slowly but surely, by means of tunneling.
After I offered my hypothesis...因為有個『之後』,表示他把他的理論講完了,然後用個時間記號區隔,因此不是我們要的內容。
premise1: Cosmic rays (high-energy protons and other particles) might trigger the synthesis of simple molecules, such as interstellar formaldehyde, in dark clouds of interstellar dust.
premise1: Afterward complex organic molecules would be formed, slowly but surely, by means of tunneling.
conclusion: This tunneling phenomenon at very low temperatures suggested my hypothesis about a cold prehistory of life: the formation of rather complex organic molecules in the deep cold of outer space, where temperatures usually reach only a few degrees Kelvin.
結論的定義是作者認為是對的事情,既然他認爲他是對的所以當然就有suggested字眼,因此將他放在結論上,也就是個人觀點的部分,然後前提就是去支持作者認為是對的那件事,所以就是補充說明舉例去支持結論的意思。
分配完之後再來就是要討論weaken是啥鬼,他是在削弱,表示整個辯論裡面的任何一句話(前提,結論)是可以被質疑的,讓文章內容形成錯誤描述,所以只要把選項代進去內容裡面看是否能夠讓以上三句話有瑕疵,那就是答案了,(A)選項剛好跟『前提一』講的是『相反』的事情,所以造成前提一的敘述『被質疑』了,同時再次提醒解批判性推理題時務必要先確認其連貫性(coherence),不能無中生有,選(A)
3.Which of the following best describes the hypothesis of Hoyle and Wickramasinghe as it is presented in the passage?
(A) Cosmic rays can directly synthesize complex organic molecules.
(B) The galactic clouds are the places where prebiological evolution of compounds necessary to life occurred.
(C) Interstellar formaldehyde can be synthesized by tunneling.
(D) Molecules of interstellar formaldehyde can evolve into complex organic molecules.
(E) Complex organic molecules can be synthesized from stable polysaccharides such as cellulose and starch.
解題:
H&W的觀點在最後兩句話,因為要描述他們的內容所以是找到這句話
After I offered my hypothesis, Hoyle and Wickramasinghe argued that molecules of interstellar formaldehyde have indeed evolved into stable polysaccharides such as cellulose and starch
argue表示某某人認為,所以就是describe的意思,那後面的主幹是molecules evolved into stable polysaccharides,選(D)
4.Which of the following best describes the organization of the first two paragraphs of the passage?
(A) The author cites a basic principle of classical chemistry and then describes the research from which that principle was developed.
(B) The author cites an apparent contradiction to the principles of classical chemistry and then explains the process of a chemical reaction to show there is in fact no contradiction.
(C) the author describes the role of heat in chemical reactions and then offers a detailed explanation of its function.
(D) The author presents a law of classical chemistry in order to introduce a kind of chemical reaction that differs from it and then explains the essential difference between the two.
(E) The author presents the fundamental rules of classical chemistry in order to introduce an explanation of a specific chemical reaction.
解題:
結構圖上看到有兩個反應在『對比』,表示要有一個轉折或是取反,(D) differs from,選(D)
5. The author of the passage is primarily concerned with
(A) describing how the principles of classical chemistry were developed
(B) initiating a debate about the kinds of chemical reactions required for the development of life
(C) explaining how current research in chemistry may be related to broader biological concerns
(D) reconciling opposing theories about chemical reactions
(E) clarifying inherent ambiguities in the laws of classical chemistry
解題:
這次換整篇文章問什麼,所以第三段的作者自述再加上去,反而使得一二兩段變成比喻了,所以主題是討論生物,不是物理,(C)be related to連接兩學科領域,而且有只有該選項兩個學科都出現,選(C)。
這篇如果是物理系畢業的人應該會很高興,背景知識就可以用來做題了,這也是我GRE閱讀的最後一篇內容,我也不知道做65篇夠不夠用,但也算是一個階段性的任務,再做下去馬車都變南瓜了and winter is comming,感覺上東西已經開始不斷重複,因此有時候寫的反而沒有剛開始來的詳細,不過還是很樂意向大家分享解題技巧,雖不知道自己描述得好不好,有時候自己在想事情當局者迷嘛,自己想說的可能無法傳達到位,反正如果有問題再留言吧,各位考友~~加油!!


 留言列表
留言列表

 {{ article.title }}
{{ article.title }}
